Colour Trademarks in the Pharmaceutical Industry
 Posted On
Posted On
A trademark aids the customers in identifying the goods and services of one undertaking from those of others. In the present fast-paced society, conventional marks are not the only type of marks available that are worthy of obtaining trademark protection. Colours and colour combinations play a significant role in differentiating the goods in the market. A colour trademark is the one in which there is at least one colour used that performs the function of a trademark, i.e., uniquely identifying the commercial origin of goods and services. Nowadays, several countries allow the registration of a single colour trademark. A question arises when we ponder upon the relevance of colour marks in pharmaceutical products – Is there any necessity to protect colour marks when products are bought based on prescriptions by doctors? The answer is yes. Pharmaceutical companies have increasingly started to register colour marks on pharmaceutical products to indicate the source of those products as belonging to their brand. It is because of several factors – firstly, a lot of customers nowadays purchase Over-the-Counter (OTC) drugs, and trademarks assist in recognizing a specific brand, which they trust. Secondly, a colour on pharmaceutical products has come to be seen as creating a brand image, and thirdly, colour trademarks serve as a mark of the brand, which creates it. Some examples of pharmaceutical colour trademarks are as follows:
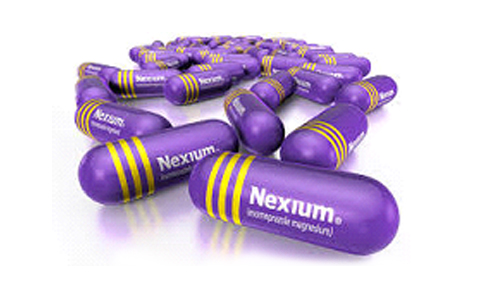
Purple for AstraZeneca’s Nexium called “The purple pill”
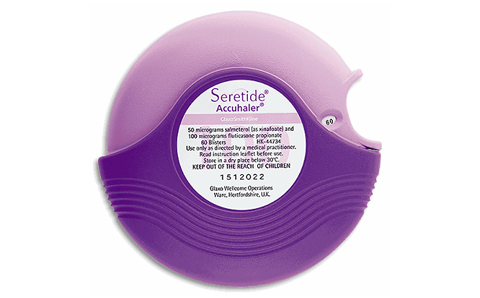
Purple for GlaxoSmithKline’s Seretide Inhaler

SK&F’s red and white Dyazide capsule
Understanding the Role of Colour Trademarks in the Pharmaceutical Industry
The trademarks for pharma goods can be classified into word marks, device marks, and trade dress. Word marks consist of drug names such as Nexium, Prilosec, Zinetac, etc. Device marks consist of the appearance colour, shape, and logo of the product. Trade Dress comprises packaging of drugs like containers, blisters, flasks, vials, etc.
Until the mid-1900s, all the prescription drugs that were in pill form were uniformly white and round. The OTC medication was also white or pastel in colour. Colours were introduced in the 1960s. By 1975, with the emergence of soft gel capsules, colours such as red, yellow, and lime green were seen. Today there are thousands of colours present in the pharmaceutical market. The colours chosen are for marketing purposes and have no bearing on the efficacy of the drug. Colour marks in the pharma sector have become a crucial branding technique because they address the visual features by distinguishing the products from those of the competitors. Colours are now being used for creating brand images, signifying the personality of the products, and differentiating them from other brands; due to which, the pharmaceutical companies spend large amounts of money today on the most attractive and appealing trade dress and marks for every new product brought to the market.
Colour trademarks can be useful for pharmaceutical products to remain in the market for an extended duration. The same becomes more vital when a brand’s immunity from generics comes close to an end towards the expiration of the patent term. The effectively registered trademarks with acquired distinctiveness may stop the generic companies from manufacturing identical-looking products. The customers may adhere to the registered products as the generic versions will look, unlike the original ones that belong to a brand that they already trust. In this manner, customer loyalty for branded medicines can be built for sustained trade in the marketplace and to uphold the market share even with stiff competition. Colour and colour combinations are a powerful way to create an emotional appeal.
The U.S.-based pharmaceutical company, AstraZeneca, in 2015 had filed a case before a Delaware court against the purple colour of the generic form of AstraZeneca’s antacid medicine, Nexium, which they marketed as “the purple pill.” The generic pill, also purple (in colour), was sold by Dr. Reddy’s Laboratories in the United States. AstraZeneca contended that this was a substantial breach of an agreement between the two companies. AstraZeneca argued that the shade of purple used was parallel to the shade of the original medicine – successfully infringing on its trademark registration for the purple medicines. Ultimately, Dr. Reddy’s Laboratories had to relaunch the generic capsules in blue colour.
Advantages of Colour Trademarks for Pharmaceutical Products

International Requirements of Registering a Colour Mark
The TRIPS Agreement lays down “Combinations of colours…shall be eligible for registration as trademarks although members may make registrability depend on distinctiveness acquired through use and members may require, as a condition of registration, that signs be visually perceptible.” Hence, countries can choose to register colour trademarks based on acquired distinctiveness and graphical representation.
In the United States, traditionally, courts were unwilling to recognize marks comprised exclusively of colours or colour combinations. It was only in 1995 that the United States Supreme Court held in the case of Qualitex Co. v. Jacobson that, “sometimes, a colour will meet ordinary legal trademark requirements. And, when it does so, no special rule prevents colour alone from serving as a trademark. However, the Court also observed that a single colour may not be intrinsically distinctive and may only be protected when it has developed a secondary meaning through use parallel to descriptive marks or words.
Countries such as Germany, Norway, Sweden, and the UK, need “display of secondary meaning or acquired distinctiveness before registering a colour per se.” The European Union Intellectual Property Office (EUIPO) also follows this rule. The UK has also acknowledged and registered colours as trademarks, for instance – “silver for anthracite briquettes,” red for the “pin of a shackle,” and “three red bands on the handle of rackets.”
The two most crucial requirements to remember for obtaining a trademark registration for a single colour mark are as follows:

Proprietors should be careful not to advertise or rely on colour as a necessary function of that particular product, as this would lead to rejection based on it being an essential feature of the trademark.
The Indian Perspective
In India, the pharmaceutical industry notably accounts for the maximum trademark registration applications among all the sectors. Colour marks are not easy to register in India, specifically single colour marks. Although the Trademark Act, 1999 does not explicitly forbid the registration of the single colour marks; showing distinctiveness in a single colour is tricky unless the colour, due to a long association with a specific mark, has come to characterize the source/origin of the product, enabling easy differentiation of the product from others in the same class. Nevertheless, single colour trademarks have been protected in India, such as the colour purple for Cadbury, colour blue for Parachute bottles, colour magenta for Telekom AG, and so on.
In India, OTC medicines and pharmaceutical wellness products are readily available without any requirement of a prescription. In OTC and wellness medicines, where the person makes his own buying choice is where trademarks play a much more significant role. A branded mark, which the customer is familiar with and trusts, has a higher possibility of being picked and helping build upon brand loyalty.
In Glaxo Group Limited vs. S.D. Garg case, the Court applied the principle of ‘likelihood of confusion’ based on the deceptive similarity of ‘Bectodine – M and ‘Betadine’ marks, trade dress, and packaging as well as the identity of colour scheme, get-up, and layout. The Court also observed that although medicines are used to treat the same ailment, this, however, does not negate the possibility of side effects.
The Manual of Trademarks, Practice, and Procedure 2015 provides the following points for registration of colour marks:
- If the applicant is claiming a combination of colours, as applied to the goods or their packaging, or as used in relation to their services, as a trademark, they should claim this by identifying the trademark as a colour trademark. Along with the exact description of the colour combination as per the International Classification System of Colours as well as supplying a graphical representation of the trademark, the applicant must also provide a concise and accurate description of the trademark on the application. The manual further gives an example of a suitable form of description for a trademark, which consists of a combination of colours applied to a pharmaceutical capsule as: “The trademark consists of a maroon colour applied to one half of a capsule at one end, and a gold colour applied to the other half, as illustrated in the representation on the application.”
- If a statement has been made in the application that the trademark consists of combination of colours only, the trademark will be regarded as colour trademark. If a particular combination of colours of packaging has become distinctive, in fact, as indicating the goods of a particular trader, there is no reason why it should not be protected by registration. However, if the colours are used not in a special or particular pattern or arrangement, it is likely to be more difficult to prove that in such cases colour would lend distinctiveness as a badge of origin.
- Wherever the exclusive right to colour is sought, weighty evidence should be necessary to overcome the objection under Section 9(1)(a) of the Trademark Act, 1999, which bars registration of trademarks that do not have a distinctive character.
- Single Colour: A single colour may be registerable as a trademark if it is very unusual and peculiar in a trade and is recognized by traders and consumers alike that it serves as a badge of origin for that class of goods.
- Combination of Colours: A combination of colours may be registrable, but this will depend on its uniqueness and how it is used. If the colours are presented as a figurative mark, then as few as two colours could be accepted; when applications consist merely of colours applied to the goods or their packaging, it will be necessary to consider how unusual the colour combination is in relation to the goods and whether, prima facie, the combination is likely to strike the relevant consumer as an indication of trade source.
Final Thoughts
As colour marks are being increasingly used by the pharmaceutical industry, one needs to comply with the trademark laws and procedures and ensure that the colour mark indicates the source of the product, that the colour is not a functional aspect of the product, and that it has acquired distinctiveness. Since a single colour lacks the inherent capability to be distinct, the standard of proof has been kept high. However, there is no exhaustive test as to whether the colour has acquired distinctiveness, and it all depends upon how the customers perceive the colour. Therefore, pharmaceutical companies need to build their brand by using distinctive trademarks, specifically colour marks. The best practices to be followed in this aspect are as follows:
- Advertisements showing that the colour has some functional advantages should be avoided.
- The colour feature should be used in a very specific manner on specific products such that it leaves many available alternative branding features for those manufacturing the same drug. For example, with Nexium, there was no advantage of making the pill purple other than making it identifiable to the brand. It left numerous other types of features and colours available to those manufacturing the same antacid pill.
- The colour feature of the drug should be marketed well.
- In case of a single colour mark, it should be registered once it has acquired distinctiveness, hence adding valuable protection to a pharmaceutical brand.
- The particular colour(s) on which protection is being sought should be defined well with the help of internationally recognized colour identification systems such as the Pantone Matching System. 👉 ✅ For more visit: https://www.kashishipr.com/
